Gel-clot Formulation
This is the simplest LAL test. The formation of a gel-clot indicates the presence of endotoxin in a sample. The method is performed in small test tubes and is read manually by inverting the test tubes. The maximum sensitivity is 0.03 EU/mL.
- Requires non-circulating water bath or dry bath incubator
- Manually read test
- Reagents of differing sensitivity available: 0.5 (STV only), 0.25, 0.125, 0.06 and 0.03 EU/mL
- May be less sensitive to interference than other LAL methods
- Is the "gold standard" for the great majority of products in the United States, European and Japanese pharmacopeia (i.e. it is the default method used to resolve a dispute)
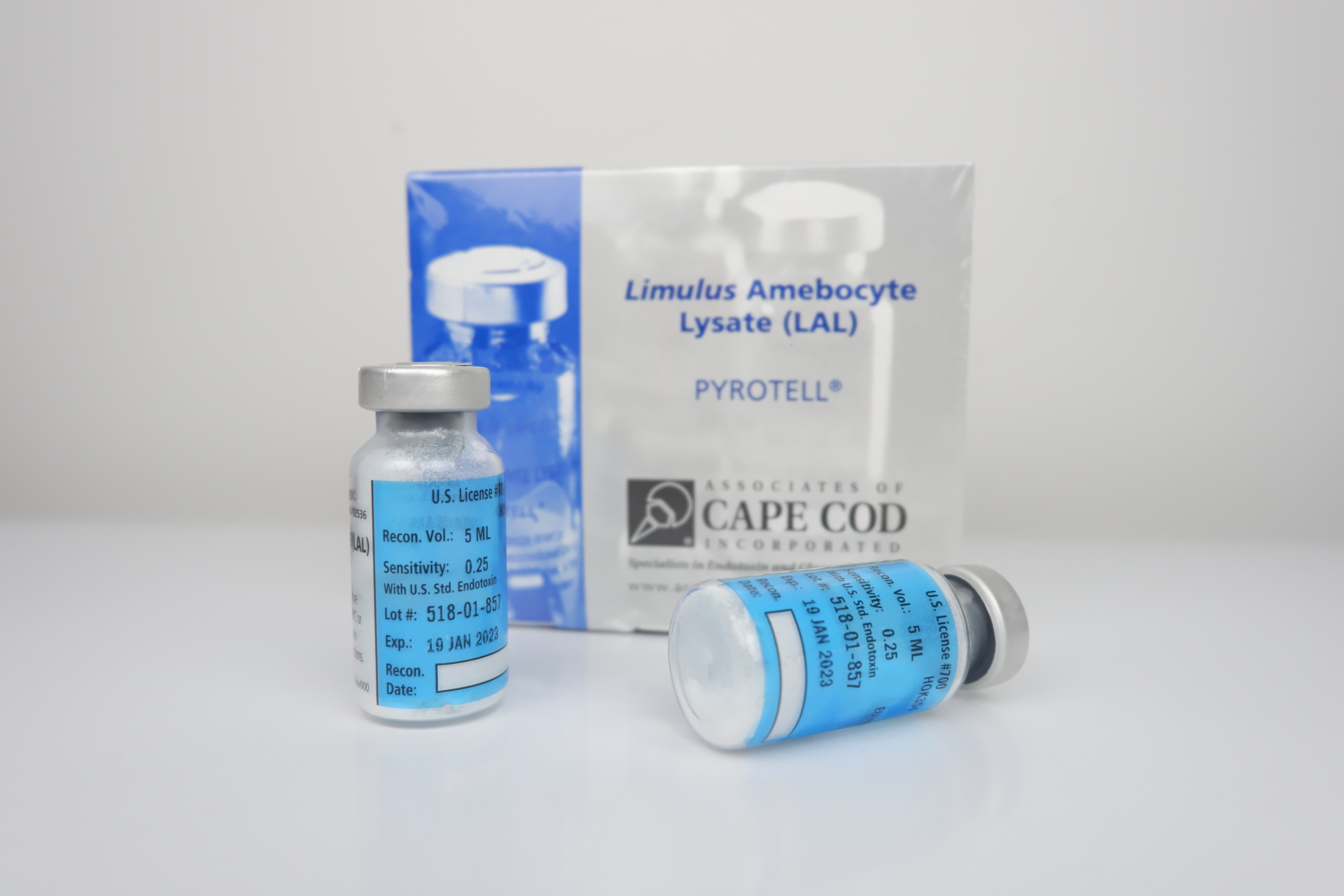
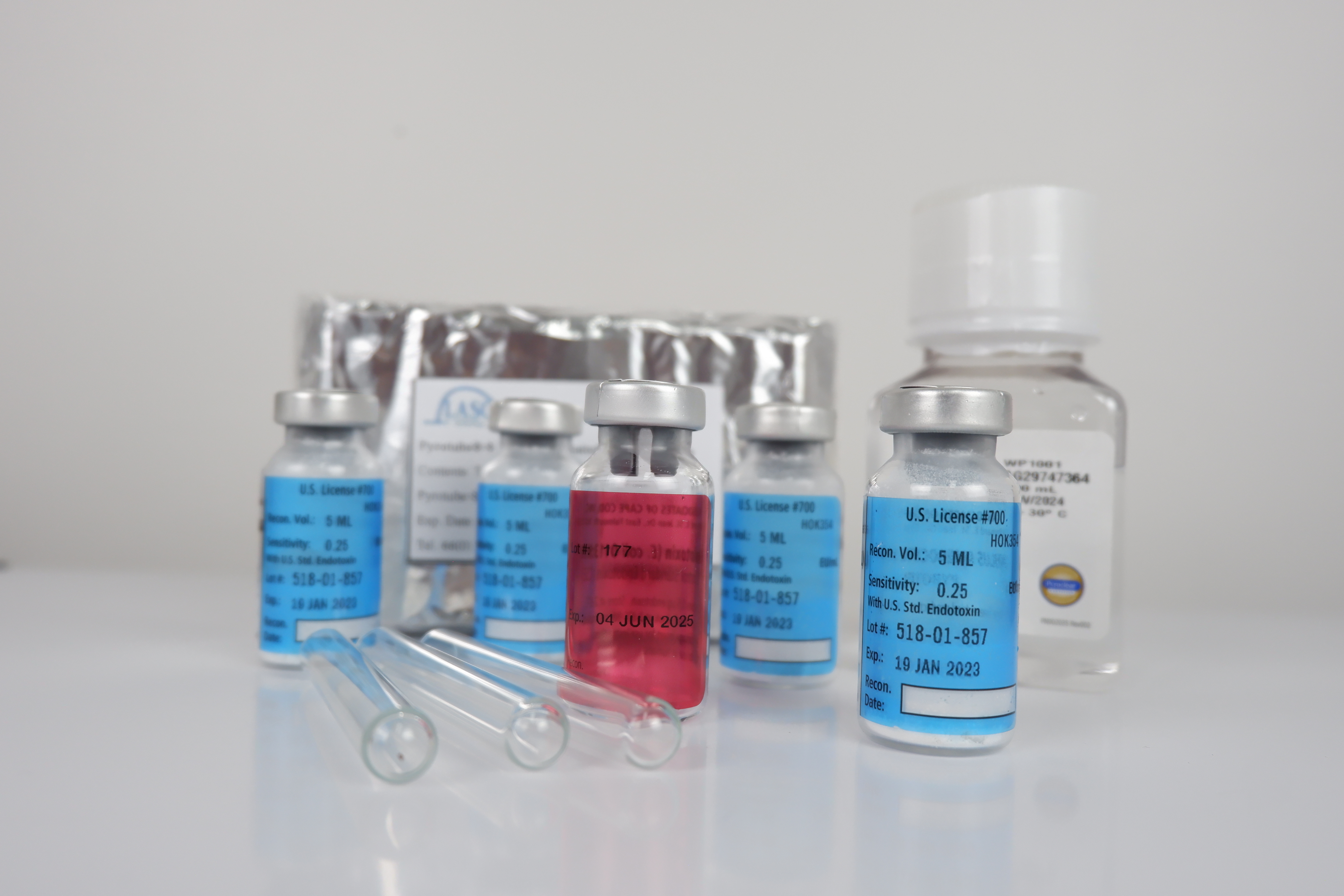
Pyrotell®
Gel-clot Formulation
Pyrotell® lysate was the first LAL gel-clot reagent licensed by the US FDA. It is easy to use and is available in both economical multi-test vials and convenient Single Test Vials (STV's). Pyrotell® lysate is a robust reagent, producing firm, easily read clots and is resistant to interfering substances. The gel-clot test does not require sophisticated capital equipment and software and is the simplest LAL test to implement.
View Product
Control Standard Endotoxin (CSE)
Gel-clot Formulation
Control Standard Endotoxin (CSE) is a widely used standard for endotoxin testing. It is a purified extract of E. coli O113:H10, the same strain used for the United States Pharmacopeia and the European Pharmacopeia reference standard endotoxin (RSE).
View Product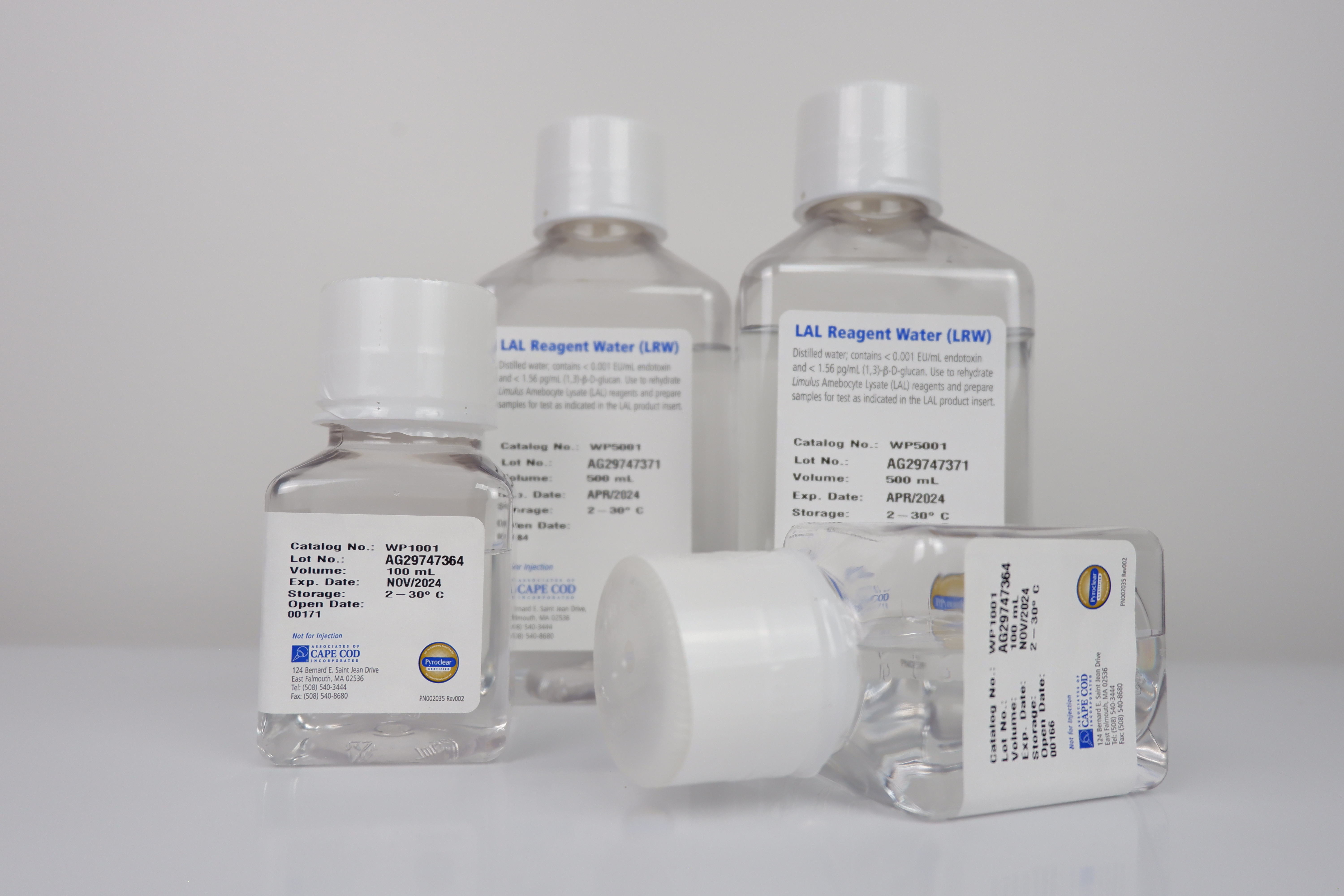
LAL Reagent Water (LRW)
Gel-clot Formulation
LRW is specially processed water intended for reconstitution of LAL reagents and CSE, and to dilute samples and standards for LAL assays.
View Product
Pyrotubes®
Labware
Our Pyroclear® Pyrotube® dilution tubes are for laboratory use only and are certified free of interfering endotoxins and glucans.
View Product